Teriflunomide
From Wikipedia, the free encyclopedia
 |
|
|---|---|
| Systematic (IUPAC) name | |
| (2Z)-2-cyano-3-hydroxy-N-[4-(trifluoromethyl)phenyl]but-2-enamide | |
| Clinical data | |
| Pregnancy cat. | Not yet classified |
| Legal status | Investigational |
| Routes | Oral |
| Pharmacokinetic data | |
| Protein binding | >99.3% |
| Half-life | 2 weeks |
| Excretion | Biliary/fecal, renal |
| Identifiers | |
| CAS number | 163451-81-8 |
| ATC code | None |
| PubChem | CID 5479847 |
| ChemSpider | 16737143 |
| UNII | 1C058IKG3B |
| ChEMBL | CHEMBL973 |
| Chemical data | |
| Formula | C12H9F3N2O2 |
| Mol. mass | 270.207 g/mol |
| SMILES | eMolecules & PubChem |
| |
|
Contents |
Mechanisms of action
Teriflunomide is an immunomodulatory drug inhibiting pyrimidine de novo synthesis by blocking the enzyme dihydroorotate dehydrogenase. It is uncertain whether this explains its effect on MS lesions.[5]Teriflunomide inhibits rapidly dividing cells, including activated T cells, which are thought to drive the disease process in MS. Teriflunomide may decrease the risk of infections compared to chemotherapy-like drugs because of its more-limited effects on the immune system.[6]
It has been found that teriflunomid blocks the transcription factor NF-κB. It also inhibits tyrosine kinase enzymes, but only in high doses not clinically used.[7]
Activation of leflunomide to teriflunomide
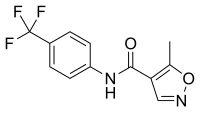 →
→ ↔
↔
The structure which results from ring opening can interconvert between the E and Z enolic forms (and the corresponding keto-amide), with the Z enol being the most stable and therefore most predominant form.
See also
See Leflunomide for information on pharmacokinetics, side-effects, contraindications and other data.References
- ^ Magne D, Mézin F, Palmer G, Guerne PA (2006). "The active metabolite of leflunomide, A77 1726, increases proliferation of human synovial fibroblasts in presence of IL-1beta and TNF-alpha". Inflamm. Res. 55 (11): 469–75. DOI:10.1007/s00011-006-5196-x. PMID 17122964.
- ^ ClinicalTrials.gov Phase III Study of Teriflunomide in Reducing the Frequency of Relapses and Accumulation of Disability in Patients With Multiple Sclerosis (TEMSO)
- ^ "Sanofi-Aventis’ Teriflunomide Comes Up Trumps in Two-Year Phase III MS Trial". 15 Oct 2010.
- ^ Gever, John (June 4, 2012). "Teriflunomide Modest Help but Safe for MS". medpage. Retrieved June 04, 2012.
- ^ H. Spreitzer (March 13, 2006). "Neue Wirkstoffe - Teriflunomid" (in German). Österreichische Apothekerzeitung (6/2006).
- ^ Dr. Timothy Vollmer (May 28, 2009). "MS Therapies in the Pipeline: Teriflunomide" (in English). EMS News (May 28, 2009).
- ^ Breedveld, FC; Dayer, J-M (November 2000). "Leflunomide: mode of action in the treatment of rheumatoid arthritis". Ann Rheum Dis 59 (11): 841–849. DOI:10.1136/ard.59.11.841. PMC 1753034. PMID 11053058.
| [hide]
|
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intracellular (initiation) |
|
||||||||||||||||||
| Intracellular (reception) |
|||||||||||||||||||
| Extracellular |
| ||||||||||||||||||


Nessun commento:
Posta un commento
https://www.youtube.com/channel/UCgPcIDxlLO6mMkmsRYHDw4g
Nota. Solo i membri di questo blog possono postare un commento.